Ok. Here's actual numbers for people to chew on.
DEF is added for pollution control, not engine performance. The main thing is NOx (Nitrous Oxides). This is NO2 and NO3 (and some NO). These compounds, when exposed to the ambient air, create 2 things: acid rain and Ozone. For acid rain, water present in the ambient air will form Nitrous or Nitric Acid (HNO2 or HNO3, respectively).
For Ozone, you need 3 things (much like fire): NOx, UV rays and Hydrocarbons. UV is from the sun; can't stop that. Hydrocarbons are from ANYTHING. Fun fact: Reagan once joked that to solve the air pollution issue, we should cut down all the trees. In one way, he's
technically correct. If you go into a pine forest in the summer, there are higher concentrations of HC than a city port. One of the worst HC is Isoprene, which is naturally emitted via photosyntesis. NOx is ONLY formed during combustion. This can be engines, furnances or fires. Yes, forest fires can create large amounts of NOx, which then goes with the HC that are being emitted to create large amounts of Ozone.
So in the US, the Ozone limits has been reduced to improve air quality. Ozone is the major issue with haze and air quality (amoung other things). The reaction to make Ozone is based upon having those three items above. You remove one of them, again much like fire, it goes down or away. That's why the Ozone rate goes away at night or dusk.
Since HC are emitted naturally, we can't really regulate those. We do limit the emissions, but people tend to get pissy about that (See the 0000 regulations for O&G wells and such). So the one people can control is NOx.
For engines, the higher you can get the cylinder temperature, the more thermally efficient it is. For gasoline engines, that's why MPG went up in the 90's then back down. They ran the engines hotter and you can make the engine leaner and pull out more MPGs. BUT your NOx creation goes off the charts at hotter flames (aka hotter cylinders). So to cool the engine, you put more gasoline in and that tampers NOx generation, but sacrifices MPG. The EXACT thing happens with Diesel.
But the issue with Diesel is that you get REALLY hot inside to ignite vs. gasoline. Especially when you go to HP systems; the temperature really goes up. So now I have this great efficiency engine, but making massive amounts of NOx. And with it, really bad air.
https://dieselnet.com/standards/us/hd.phpGo down to Table 1 (can't image it at work, so you'll have to read it).
In 1988, the NOx limit was 10.7 grams of NOx per bhp hr engine size. So if you had a 100 bhp engine, you could allow 107 g/hr of NOx from the engine. In 1990, the limit was reduced to 6; the same engine now can only emit 60 g/hr (56% reduction). Then in 1998, it was reduced to 4.0. Then in 2007, it was reduced to 0.2 g/bhp hr. In 2024, the limit was reduced to 0.05 and in 2027, it is proposed to go to 0.035.
So the same 100 bhp engine will only be allowed to emit 0.35 g/hr of NOX vs. now only emitting 2 g/hr of NOx (2024) vs. 107 in 1988.
The only way, industrially, that you remove NOx and SOX is with an SCR (Selective Catalytic Reduction) system. You can see this on gas turbines at power plants. See below:
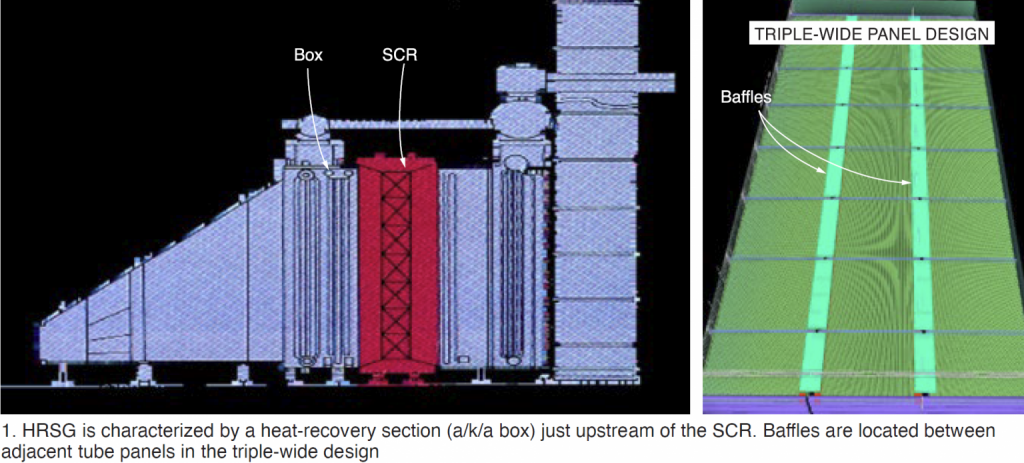

This works by using Ammonia (NH3) and allowing the NOx and NH3 to react to convert the NOx back to N2 and O2 and water over a catalyst bed. So to 'steal' the technology from industrial plants, the only way to get Diesel engines down is to add NH3. This is done with Urea (NH2CONH2) in water. When heated, the Urea breaks down into Ammonia (NH3) and then reacts with the NOx from the engine to hit the limits.
The only other options are: stop using Diesel (unpractical at this time) or limit the number of engines in the US. Now, we can talk about the Ozone limits, which has been reduced from 0.08 ppm in 1997 to 0.075 ppm in 2008 to 0.07 ppm in 2015 (80 ppb to 75 to 70 ppb).
So there is a reduction in MPG in engines to reduce NOx. But the other option to reduce air pollution is to put limits on engines. And since most people in the US think that's not going to fly, you have to make the combustion cleaner.
Interesting paper on Ozone in Houston:
https://acp.copernicus.org/articles/16/14463/2016/acp-16-14463-2016.pdf - Ozone in the morning is driven by VOC (Hydrocarbons) vs. NOx in the afternoon.
Good chemical background on Ozone:
https://projects.iq.harvard.edu/files/acmg/files/intro_atmo_chem_bookchap12.pdfNOx Information:
https://www3.epa.gov/ttncatc1/dir1/fnoxdoc.pdf - Has good info on various control technologies for both internal and external fires.
~egon












